Sulfur Burners vs. Acid Injection vs. Elemental Sulfur (Soil): The Practical Winner for High-Alkalinity Irrigation Water
Contents
Contents
If bicarbonates are the driver, the fastest path to better root-zone chemistry is treating the water—today, not next season.
Introduction
Growers describe the same pain in different words: “My pH is too high,” “nutrients don’t show up,” “emitters plug,” or “the soil keeps drifting alkaline.” The goal is almost always the same—better root-zone performance: nutrient availability, consistency, and crop response.
Here’s the distinction that changes what “the right solution” looks like:
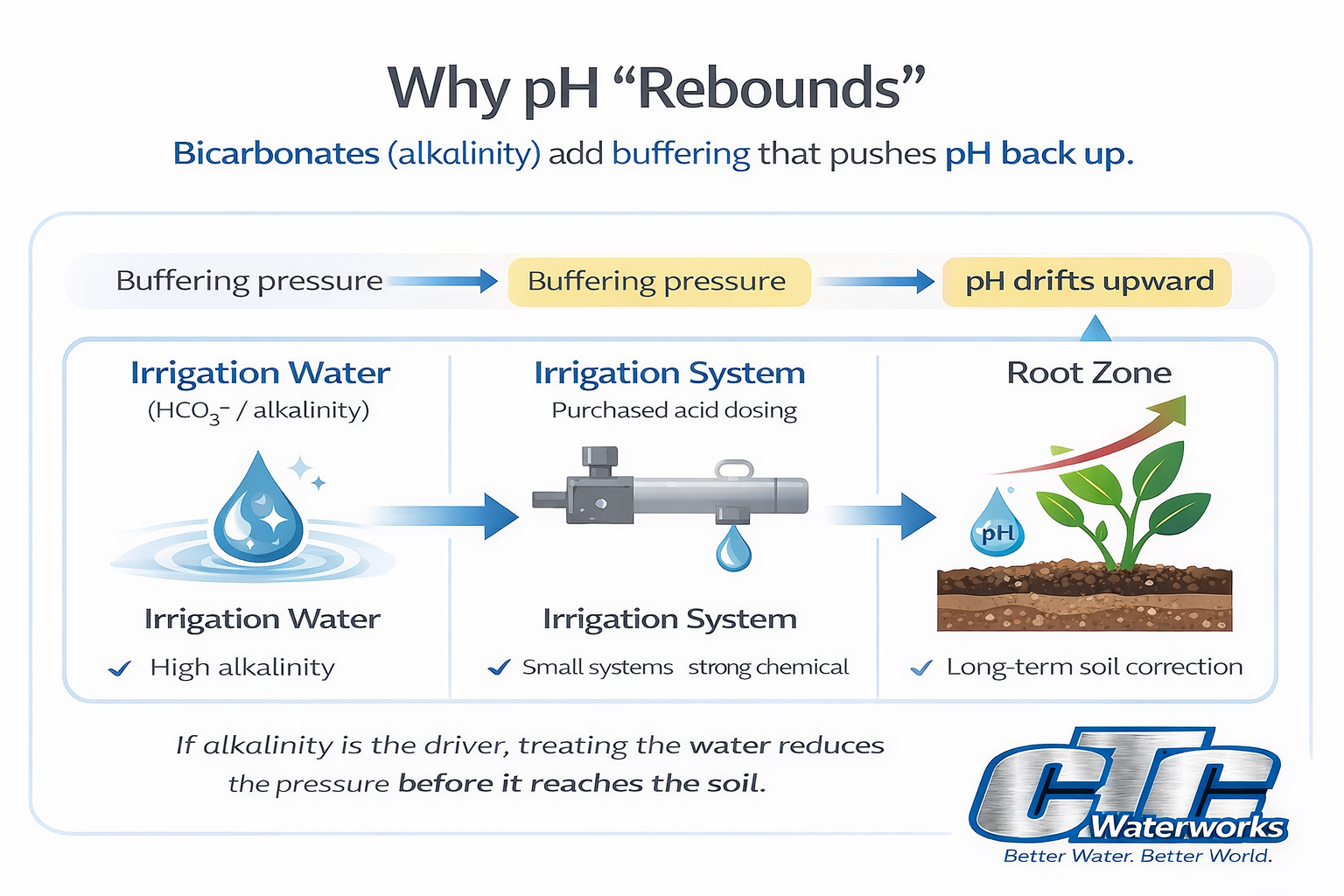
- Soil pH problems often have a water chemistry cause. Irrigation water containing bicarbonates can act like a slow liming source over time, increasing soil pH.
- If bicarbonates (alkalinity) are the driver, you’re not only fighting a pH number—you’re fighting buffering capacity that keeps pushing pH back up.
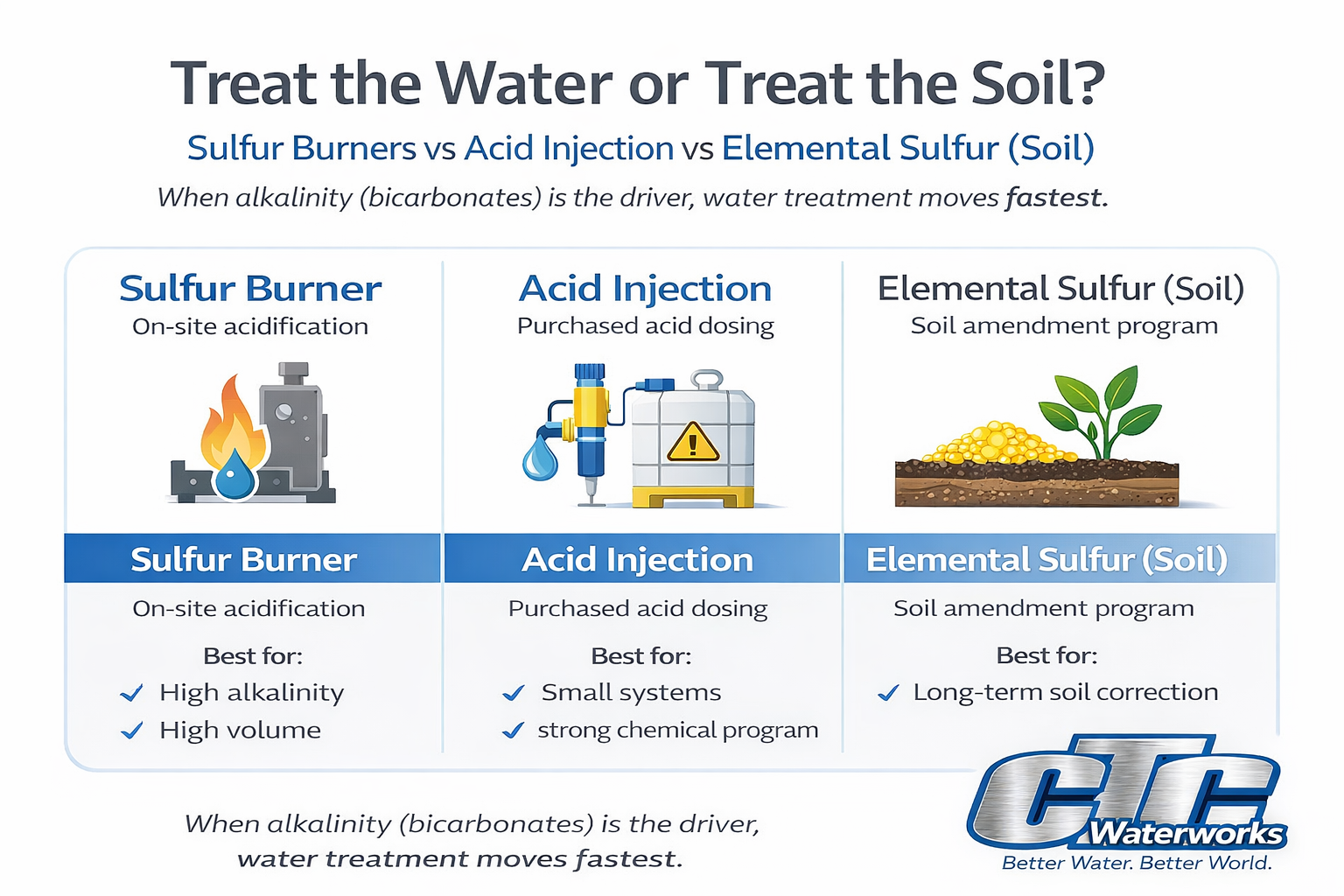
That’s why these three options get compared in the same conversation:
- Acid injection (a category: sulfuric, phosphoric, nitric, citric, etc.)
- Sulfur burners (on-site generation of sulfurous acid in the irrigation stream)
- Elemental sulfur applied to soil (a soil acidification program)
Learn how Sulfur Burners work: Sulfur Burners
TL;DR (60-second plan)
If your irrigation water has meaningful alkalinity (bicarbonates), treating the water is usually the fastest and most controllable lever for improving root-zone chemistry this season.
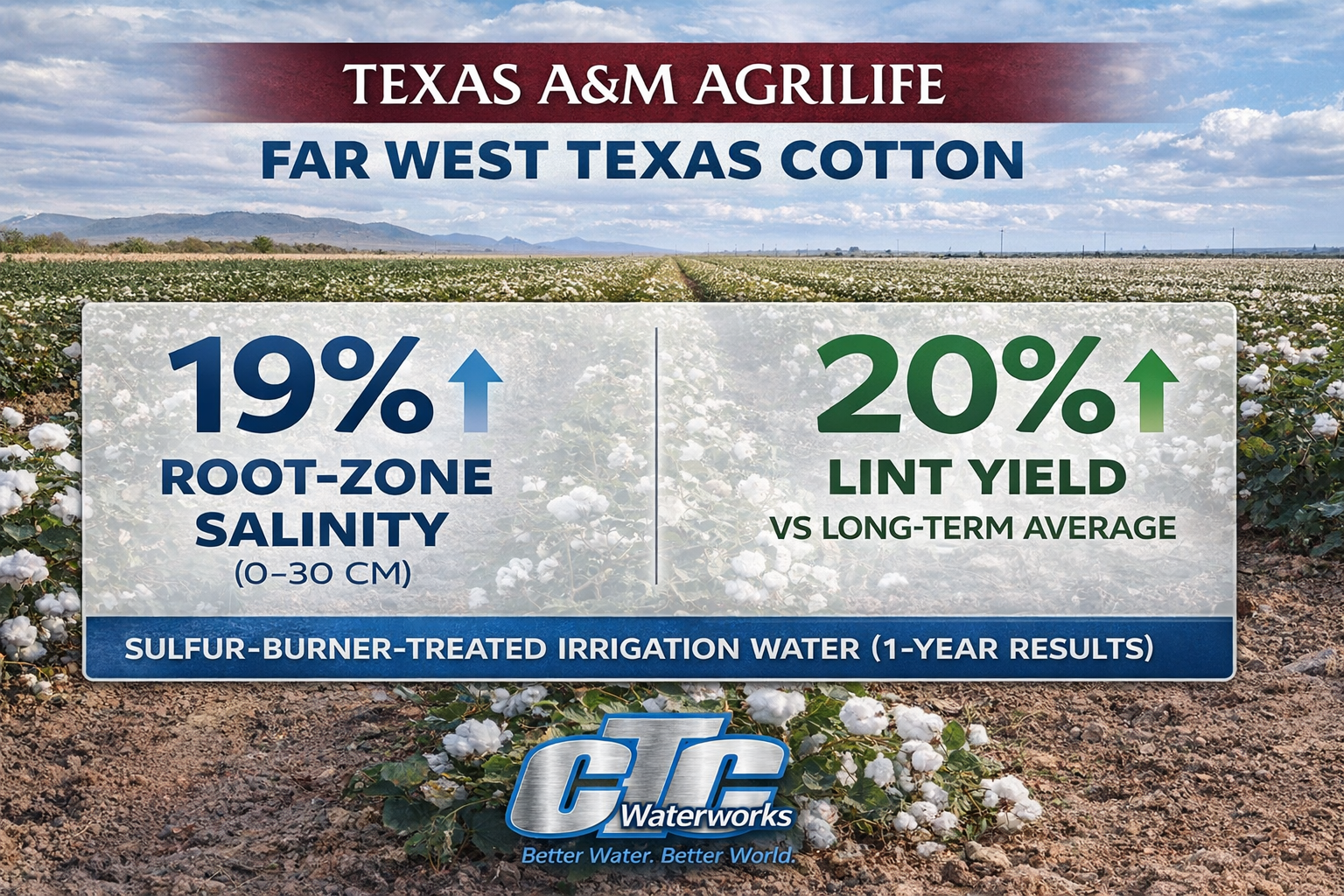
- Sulfur burners are often the practical winner in high-alkalinity field cases because they reduce alkalinity before the water hits your irrigation system and soil—without committing your team to ongoing bulk corrosive liquid acid receiving, storage, and transfer.
- Acid injection can work well, but many farms find the logistics, audit burden, and day-to-day handling of bulk corrosive liquids is the real barrier.
- Soil-applied elemental sulfur is not a water-treatment tool. It can play a role in specific situations (usually localized or pre-plant objectives), but in bicarbonate-driven scenarios it’s typically slower and less direct than treating the water that’s causing the pressure.
Important clarification
When this article says “elemental sulfur,” we mean elemental sulfur applied directly to the soil as a soil amendment. We are not talking about the elemental sulfur feedstock used in a sulfur burner. Same raw material—different application, different timeline, different outcome.
1) Do sulfur burner users still want elemental sulfur in the soil?
In most bicarbonate-driven scenarios, not really—and this is where a lot of confusion shows up.
If irrigation water is high in bicarbonate, the water keeps re-applying alkalinity pressure to the root zone every irrigation. That means you can be doing soil work while the water continues pushing conditions the other direction.
Water treatment addresses that pressure immediately. Soil-applied elemental sulfur does not.
In real operations, you typically see one of these patterns:
Pattern A: “Pick one” (common when water alkalinity is the main driver)
Growers choose a water-treatment strategy because they don’t want to wait months for a soil amendment to convert—and because treating the water stops the ongoing alkalinity load at the source.
Pattern B: Soil sulfur for a specific, limited objective (less common, but real)
Soil-applied elemental sulfur may still make sense when the need is localized (small soil volumes, banded zones, pre-plant objectives) or when only a minor correction is needed and a full water-acidification program would be overkill.
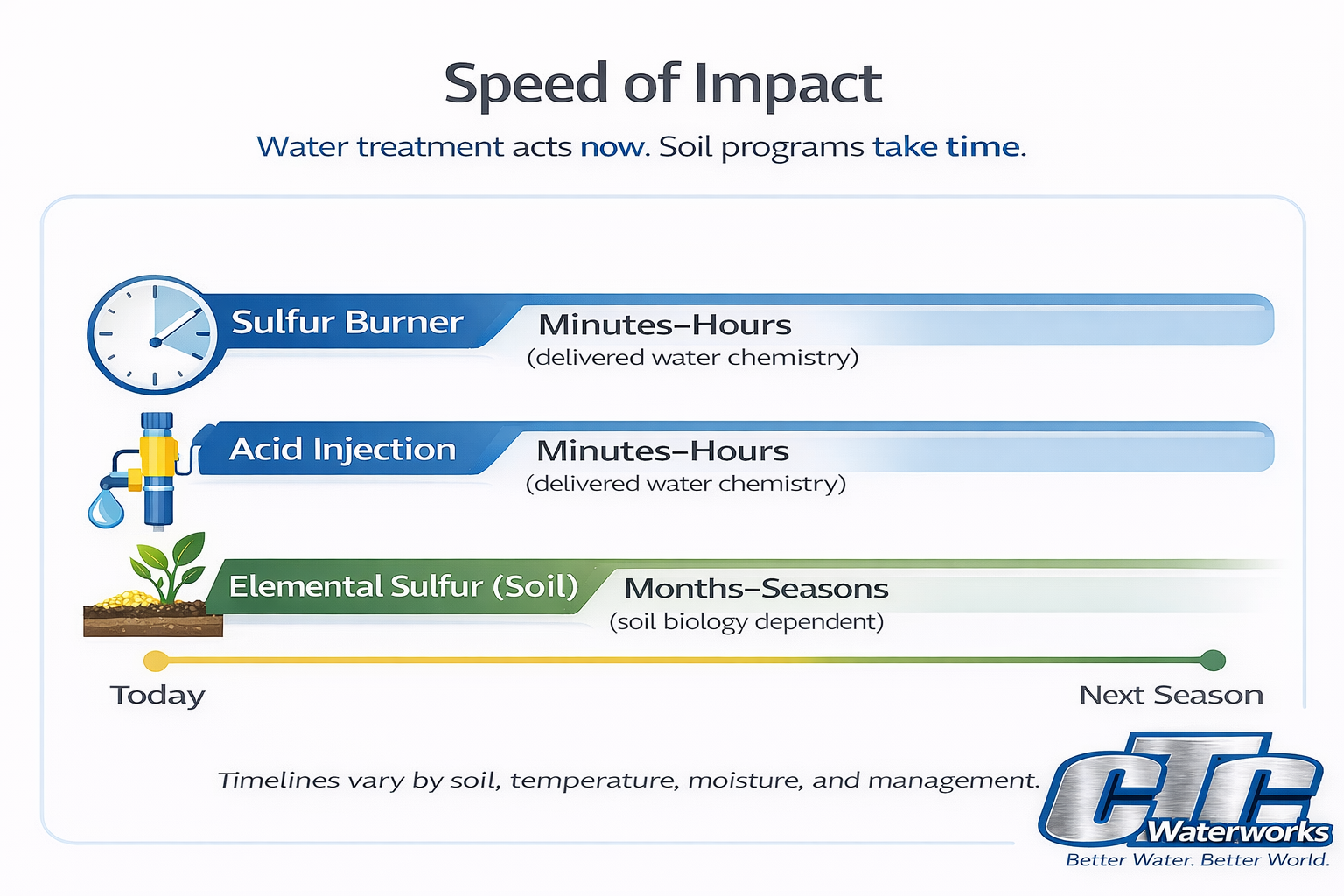
Key point: If the timeline is this season and alkalinity is the driver, treating the irrigation water is the lever that moves fastest—and usually makes the biggest difference.
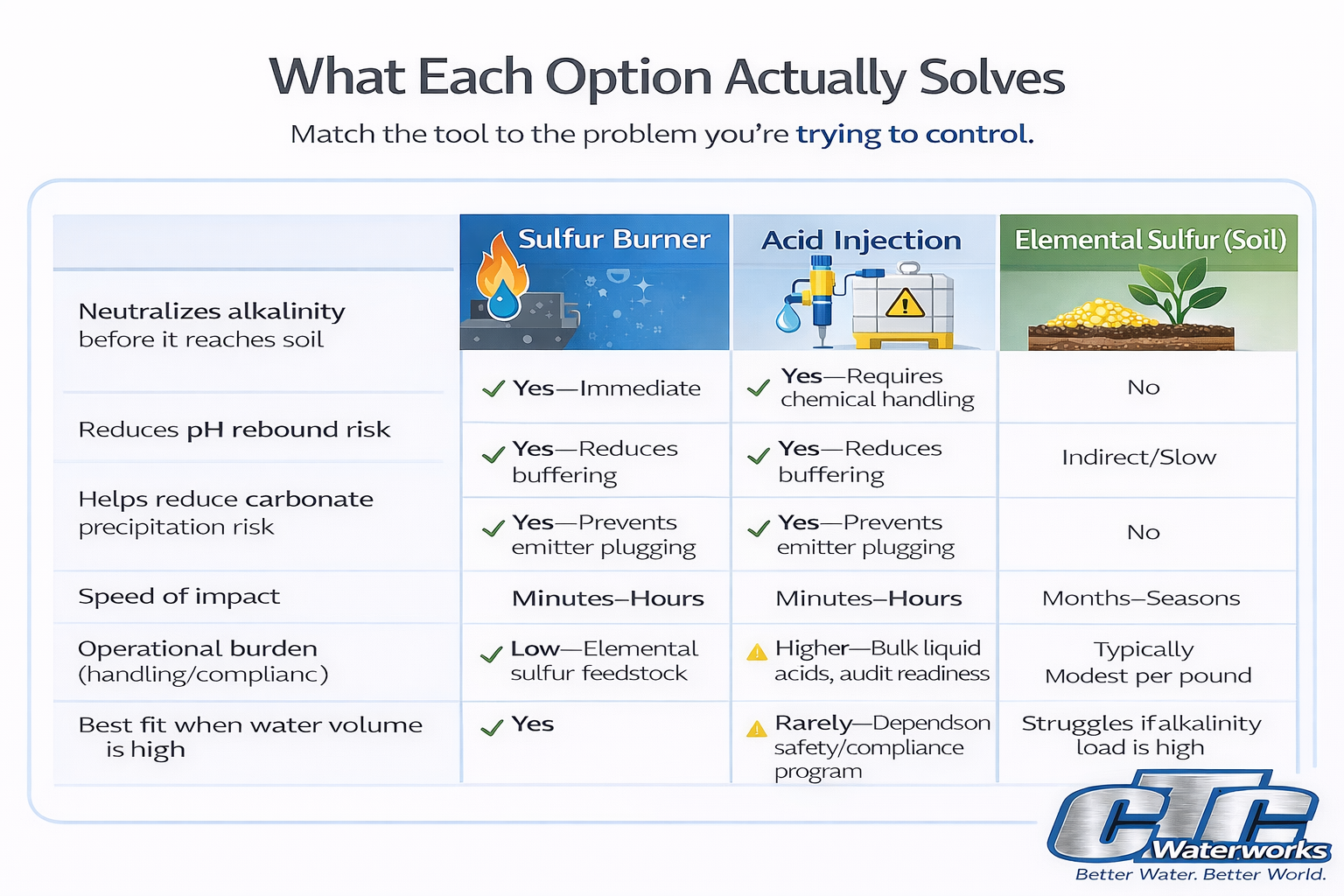
2) What each option actually solves
Option 1: Sulfur burners (sulfurous acid generated on farm)
What it is
A sulfur burner generates sulfur dioxide (SO₂) by burning elemental sulfur and dissolves it into irrigation water to form sulfurous acid in the water stream. The practical outcome is water acidification that helps neutralize alkalinity before the water reaches the irrigation system and soil.
Why it wins in many high-alkalinity field cases
- Immediate impact on delivered water chemistry
If bicarbonates are pushing pH back up, the best leverage is reducing alkalinity before the water hits your irrigation system and soil.
- Simpler operating model than many bulk acid programs
Much of the burden with acid injection isn’t the chemistry—it’s the day-to-day reality of receiving, storing, transferring, and managing bulk corrosive liquid acids. Sulfur burners shift that profile away from routine bulk acid tote handling while still delivering water acidification.
- A better fit when volumes are high
If you run big water for long seasons, “soil-only” approaches often struggle because alkalinity keeps arriving every irrigation. Water treatment scales with the water use that is creating the problem.
Organic note (for certified organic operations)
Organic requirements vary by certifier and by your Organic System Plan. In most cases, we see sulfur burners as the preferred organic pathway for irrigation water acidification because they avoid running an ongoing bulk liquid acid program on farm—while still addressing alkalinity at the source. Always confirm allowances with your certifier before implementing any program.
Operating and safety considerations (field-relevant, not alarmist)
Most sulfur burner installations are outdoors or in open-air configurations. In those common setups, “room ventilation” is typically not the deciding factor.
The real-world comparison is about what your team interacts with day to day:
- Bulk acid injection programs often require routine receiving, storage, transfers, spill-response readiness, and documentation around corrosive liquids.
- Sulfur burners generally reduce exposure to bulk corrosive liquid handling, while requiring disciplined operation of a controlled SO₂ generation process.
The right way to run a sulfur burner is to treat it like any other industrial process:
- Install outdoors/open-air where feasible and site it away from people and high-traffic work areas
- Follow manufacturer startup/shutdown procedures and maintenance intervals
- Train the team on what “normal” looks like and what to do if anything is abnormal
- Keep operating controls simple, documented, and repeatable (especially for audit/food safety alignment)
This is why many farms find sulfur burners safer, easier to manage, and a better food-safety fit than living in the routine acid tote business—without pretending there is “no risk.”
Option 2: Acid injection (sulfuric, phosphoric, nitric, citric, etc.)
What it is
Acid injection introduces a purchased acid into the irrigation water to reduce pH and neutralize alkalinity (bicarbonates/carbonates). It is commonly used to manage bicarbonates and reduce carbonate precipitation risk in irrigation systems.
Common acids used
Growers typically hear sulfuric, phosphoric, nitric, and citric discussed in acidification programs. Each choice can carry tradeoffs—nutrient “side effects” (adding N or P), fertigation compatibility considerations, and different hazards and handling requirements.
Why it can be a non-starter for audit/compliance teams
Many operations aren’t rejecting acid injection because it doesn’t work. They reject it because they don’t want a bulk corrosive liquid storage and transfer program on site. That often means documented training, PPE procedures, secondary containment, emergency response planning, transfer controls, signage, and ongoing oversight.
If you already run a mature chemical management program, acid injection can be a workhorse. But for many farms, the compliance cost and operational overhead is the real barrier.
Option 3: Elemental sulfur applied to soil (soil program, not water treatment)
What it is
Elemental sulfur applied to soil is converted by soil biology over time into acidity, which can lower soil pH gradually.
Where it fits (the realistic use cases)
Soil-applied elemental sulfur is best viewed as a targeted tool when:
- You need localized soil pH adjustment (small soil volumes, banding, specific zones)
- You’re doing a pre-plant correction with enough lead time
- Only a minor correction is needed and a full water-acidification program would be overkill
Where it does not replace water treatment
Soil-applied elemental sulfur does not change the chemistry of the water you’re applying today, and it does not stop bicarbonate alkalinity from entering the system at each irrigation. If alkalinity is the driver, this is why many growers move away from soil sulfur once water is being treated—because it’s slower and less direct than stopping the alkalinity at the source.
3) CTC perspective: why sulfur burners are often the practical winner
If your irrigation water has meaningful alkalinity load and your goal is improved root-zone chemistry this season, sulfur burners are often the most direct and practical solution because they:
- Act immediately on delivered water chemistry
- Scale well with high seasonal water volume
- Avoid the continuous burden of receiving, storing, and transferring bulk corrosive liquid acids
- Support a clean audit narrative: eliminating routine bulk acid totes/transfers while operating with defined on-farm controls
Acid injection can be effective—and in some cases cost-effective on a pure chemical basis—but the compliance and logistics overhead is frequently the deciding factor.
4) Economics: a relatable real-world example
The honest way to view the economics is to focus on what actually drives cost: alkalinity load and seasonal gallons treated.
Here’s a common, relatable scenario:
- Water test: alkalinity 200 mg/L as CaCO₃, pH ~7.8–8.2
- Flow: 800 GPM typical (large block, long sets)
- Season runtime: 12 hours/day for 120 days
- Treatment goal: reduce alkalinity by ~120 mg/L as CaCO₃ (enough to materially reduce “pH rebound” in many systems)
At that flow and runtime, you treat roughly 69 million gallons of water in a season.
If you translate that example alkalinity reduction into an “acid equivalent” (for illustration only), you are often in the neighborhood of ~5,000 gallons of concentrated sulfuric acid equivalent across the season—and that number can move up or down depending on your exact water chemistry, targets, and operating setpoints.
That’s why high-volume farms discover the real decision isn’t “does acidification work?”—it’s whether they want the season-long reality of:
- routine bulk acid receiving and storage,
- transfers/totes, containment, PPE, documentation, and audit controls,
- plus all the logistics that come with it,
or an on-farm operating model built around sulfur feedstock + energy + maintenance with defined operating procedures.
Key takeaway: Instead of claiming “X is always cheaper than Y,” the credible approach is: your water test + flow range + season runtime determines the demand. Then you compare operating models side by side.
If you want a simple baseline comparison for your actual case, try the Sulfuric vs. Sulfur Burner Cost Calculator
5) Decision guide
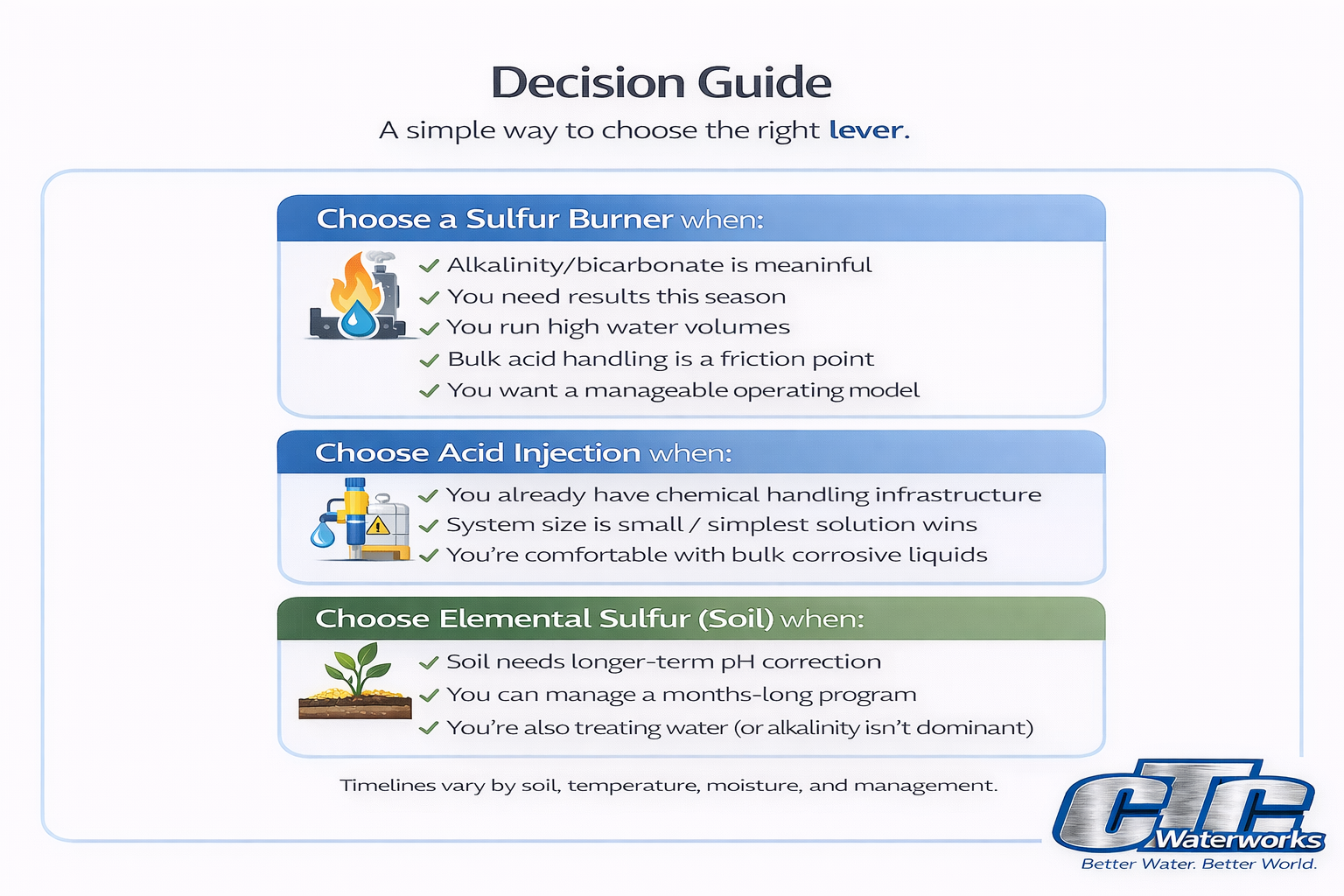
Choose a sulfur burner when:
- Your water has meaningful alkalinity/bicarbonate and pH rebounds
- You need results this season
- You run high water volumes
- Bulk liquid acid handling is a safety/audit/compliance friction point
- You want a more operationally manageable program than routine acid receiving, storage, and transfer
Choose acid injection when:
- You already have strong chemical handling infrastructure and a mature compliance program
- Your system is small enough that the simplest solution wins
- Your operation is comfortable managing bulk corrosive liquids as an ongoing practice
Choose soil-applied elemental sulfur when:
- You only need a minor or localized soil pH adjustment (small zones/banding)
- You have time for gradual change (typically months) and you’re correcting a specific soil objective
- Your water alkalinity is not the dominant driver (or your water is already being treated and you’re targeting a narrow soil zone)
Closing
If bicarbonate alkalinity is pushing your root zone out of range, treating the irrigation water is often the fastest, most controllable lever you can pull.
Acid injection works, but many farms underestimate the operational and audit burden of a bulk corrosive liquid program. Sulfur burners often win not because the chemistry is “magical,” but because the operating model is a better fit for high-volume irrigation—and for teams that want immediate results without living in the acid tote business.
Found this helpful? Let's discuss it!
Share your thoughts and experiences on LinkedIn